Advanced Solutions for the Life Sciences Industry
From highly-precise small parts handling, laser integration, and assembly automation, PAR’s solutions meet the unique needs of life science manufacturing with special expertise in medical device automation and manufacturing.
Download information about PAR’s automated assembly solutions.
Key Capabilities for the Life Sciences Industry
Software Development & DataMate
DataMate by PAR Systems is a PLC data collection and visualization tool intended for engineers to better understand the operation of a machine. It facilitates collection of PLC tags on change and/or at specified intervals. The included DataMate Dashboard facilitates analysis of collected data for calculation of machine operational equipment efficiency (OEE) and assessment of part measurements and machine alarms.
DataMate can be installed on a machine PC or on a laptop. It retrieves data from a configurable SQL server that can be hosted locally or on your company’s network. This allows engineers to review and track PLC data without requiring PLC programming experience.

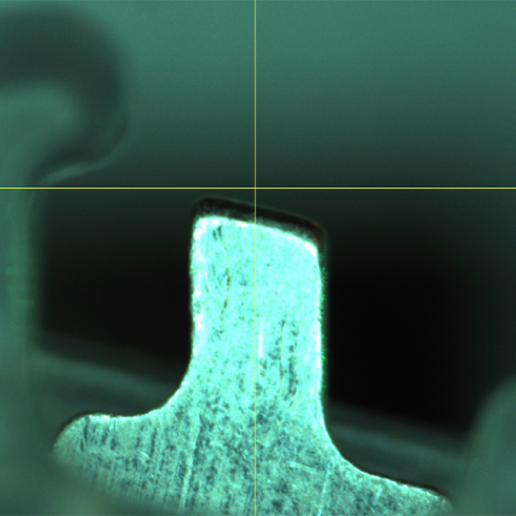
Small Parts Precision Handling
As a leader in small parts precision manufacturing, we build machine systems that deliver incredible results, particularly in small, micro-scale applications. For decades, we have pioneered technology in this space and focused on creating systems that work in the most challenging cases. In one such case, our solution performed singulation of spheres less than 300 microns in diameter, within 5 microns of positional accuracy.
Laser Integration
PAR’s laser automation capabilities include cleaning, cutting, drilling, scribing, welding, ablation, milling, inspection, and processing. Depending on the need, we can integrate multiple laser types to ensure the right option for each application. We are experts at automating any laser processes and can help you find the right laser solution for medical device manufacturing or other applications for the life sciences industry.
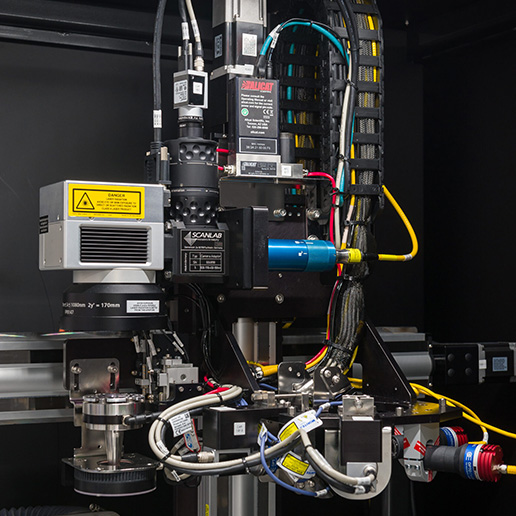
Additional industry-leading capabilities in life science.
The PAR Advantage
PAR has over 20 years of experience in the design, installation, and maintenance of complex solutions for the life sciences industry. Our focused efforts ensure the best possible performance in these three key areas:
Size
Nobody does micro parts precision handling like PAR. For various applications, we have achieved:
- Singulation of spheres that have diameters less than 300 microns and placement within 5 microns positional accuracy
- Laser drilling of 75-micron holes
- Singulation and processing of wires smaller than a 0.001″ OD (~25 microns)
Complexity
Our custom approach to each project allows us to tackle even the most complex applications. Our solutions have performed:
- Flex-feeding and laser welding of metal brackets with 96 unique SKUs
- Drilling 75-micron OD holes into platinum domes via femto laser and seven-axis scanhead, with systems running at 99% yield
- Automated cable set assembly, including identification of each conductor by color, stripping, tinning and brazing to the appropriate bulkhead location
Agility
We balance the unique needs of our customers with quick turnaround times to get our solutions up and running, providing efficiency and quality results in a timely manner. For example, we:
- Designed and build a robotic adhesive dispense system in five weeks (from purchase order to shipment) for COVID-related application
- Commissioned a custom bore inspection system in six weeks (including CE compliance testing) in response to a product recall for a medical device company
Our expertise shows in each element we craft.
"High quality systems and excellent systems integration. Better than others I have worked with."
Mfg. Engineer
Leading Medical Device company
Certifications and associations where it matters.
